- Posted By Dr. Anuranjan Bist
- Comments 0
Depression is common, but the experience of it is never “common.” It can flatten motivation, steal sleep, distort relationships, and make even simple decisions feel like dragging a boulder uphill. Globally, hundreds of millions of people live with depression. The World Health Organization estimates about 332 million people have depression worldwide.
And here’s the part that frustrates many patients the most: even when you do everything right – therapy, lifestyle changes, medication trials – relief can still feel slow, partial, or out of reach. In the large STAR*D study (often cited because it reflects “real world” treatment), the remission rate after the first SSRI step was about 28%. That means a lot of people are left asking: Why didn’t it work for me?
This is where the conversation has shifted in modern psychiatry, from only “serotonin and mood” to glutamate depression and NMDA receptor roles in brain health. This shift helps explain why ketamine can work rapidly for some people, especially when SSRIs fail.
What is glutamate depression and what does it mean in real life?
Glutamate is the brain’s primary excitatory neurotransmitter, it’s involved in learning, memory, attention, and the way brain circuits communicate. If serotonin is often described as a “mood chemical,” glutamate is more like the brain’s communication infrastructure.
When people use the phrase glutamate depression, they’re pointing to a newer model: depression is not only about mood chemicals being “low” or “high.” It can be about how well brain networks talk to each other, and whether key circuits have lost flexibility after chronic stress.
In everyday language, glutamate depression looks like this:
- You can understand what you should do, but you can’t access the energy to do it
- Your thoughts loop, and the loop feels louder than logic
- Motivation and pleasure don’t return even after “good news”
- Your brain feels less adaptable, less responsive, less “alive”
That “stuckness” is often what people mean when they say depression feels like it’s “in the brain,” not just “in the mind.”
Why do SSRIs often help some people but fail others?
SSRIs can be life-changing for many people. But they have two built-in limitations that matter here.
SSRIs target serotonin first, but neuroplasticity later
SSRIs increase serotonin signaling relatively quickly, but symptom relief often takes weeks. One reason is that the most meaningful improvements may rely on slower downstream changes, like shifts in receptor sensitivity, stress hormones, inflammation signaling, and neuroplasticity.
Depression is not one uniform disorder
Depression can arise through different biological routes: trauma-related circuitry, inflammatory pathways, hormonal shifts, sleep disruption, or network “wear and tear” from long-term stress. If your dominant problem is synaptic connectivity and circuit flexibility, a serotonin-only strategy can feel like treating a network problem with a single dial.
This is why STAR*D matters: in that first SSRI step, most people did not reach remission. It’s not a personal failure. It’s a signal that we need broader models.
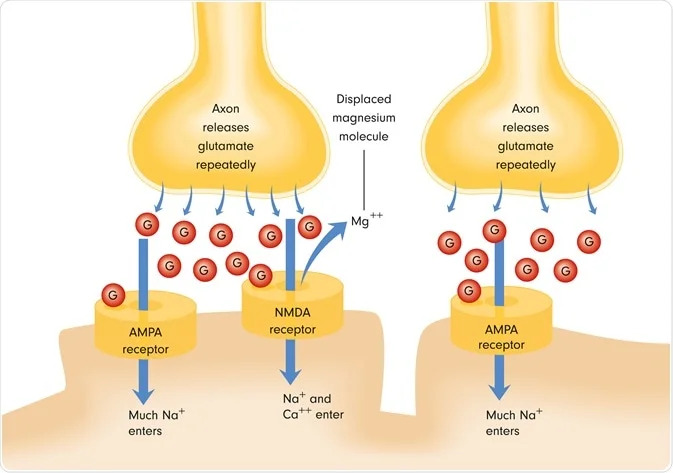
What is the NMDA receptor and why is it a turning point in depression science?
To understand ketamine, you need to know the NMDA receptor. The NMDA receptor is a glutamate receptor involved in:
- synaptic strengthening
- learning and memory
- adaptive rewiring after experience
- the brain’s ability to change (plasticity)
So when we talk about glutamate depression and NMDA receptor roles, we’re talking about the very machinery the brain uses to update itself.
In many modern models, depression is not just “sadness.” It can be a state where:
- stress has weakened synapses in mood-related circuits
- the brain becomes less flexible
- negative networks dominate attention and interpretation
- positive reward circuits stop responding
That’s why the NMDA receptor matters: it sits at the crossroads of signal, learning, and synaptic remodeling.
How does ketamine work where SSRIs struggle?
Ketamine’s antidepressant story is often called a “revolution” because it challenged a decades-old assumption: that meaningful antidepressant effects must take weeks.
Ketamine is understood as an NMDA receptor blocker (more specifically, an NMDA receptor channel blocker), and this triggers downstream changes that can rapidly affect synaptic function.
But the most useful way to think about ketamine is not “it blocks NMDA, therefore mood improves.” It’s more interesting than that.
Step 1: A reset in glutamate signaling
By altering NMDA receptor activity, ketamine can change how glutamate flows through key circuits. Many researchers describe a shift that ultimately increases effective AMPA-to-NMDA throughput, which then drives synaptogenic signaling (Aleksandrova et al., 2017).
Step 2: A neuroplasticity cascade
Downstream, ketamine is associated with pathways tied to synaptic repair and growth (often discussed in relation to BDNF and mTOR signaling in the literature).
Step 3: Rapid symptom relief in some patients
A meta-analysis of randomized trials found ketamine’s effects were significantly better than placebo at 24 hours, 72 hours, and 7 days, with reported response rates around 52% at 24 hours in the analyzed studies.
That speed matters, especially for people who have spent months (or years) cycling through treatments.
Who may benefit most from glutamate focused treatments?
Glutamate-focused approaches are most often discussed in these situations:
- Treatment-resistant depression, where multiple standard treatments have not achieved remission
- Severe depression where waiting weeks for relief feels clinically risky
- Depression with prominent cognitive symptoms, numbness, or rigid negative loops
- Patients who need a rapid “window of relief” to re-engage therapy, sleep, movement, and routine
This doesn’t mean ketamine is “for everyone.” It means it belongs in a modern toolkit for specific profiles, especially when the biology looks less like a serotonin-only issue and more like a circuit-plasticity issue.
If you’d like to explore options in a structured way, you can link this topic to your broader care plan through:
- Depression Treatment
- Neuroplasticity Based Therapies
- Treatment Resistant Depression Program
At Mind Brain Institute, such cases are approached through a holistic and individualized care framework that addresses biological, psychological, and neuroplastic dimensions of depression.
What should you know about safety and real world use of ketamine?
Ketamine is a serious medical intervention. It can cause short-term effects such as dissociation, temporary changes in blood pressure, and perceptual shifts, which is why its use is governed by supervised clinical protocols. Regulatory bodies have also cautioned against non-standard compounded products and unsupervised settings, as safety and outcomes depend heavily on how and where ketamine is administered.
At Mind Brain Institute, ketamine assisted therapy is delivered within a structured medical framework that prioritizes patient safety, clinical judgment, and long-term recovery rather than rapid symptom suppression alone.
A responsible approach includes all of the following:
- careful diagnosis, since depression subtypes respond differently
- thorough screening, including medical history, blood pressure, dissociation risk, and substance-use considerations
- monitored administration in an appropriate clinical setting when indicated
- thoughtful integration through therapy and lifestyle support to help sustain benefits
Ketamine is best understood as a neurobiological opening, not a standalone cure. The durability of improvement often depends on what follows that opening—restoring sleep, engaging in therapy, rebuilding routine, strengthening relationships, and reconnecting with meaning. When used this way, ketamine assisted therapy becomes part of a broader, ethically grounded treatment journey rather than a shortcut.
Takeaway…
Depression is not always a simple chemical imbalance, and for many people, it reflects disrupted brain circuits and reduced neuroplasticity rather than serotonin alone. Understanding glutamate depression and NMDA receptor roles helps explain why ketamine can offer meaningful relief when traditional treatments fall short. Used responsibly, ketamine opens a window for the brain to regain flexibility, making therapy, sleep, and daily routines more accessible again. At Mind Brain Institute, this understanding is applied through a careful, holistic, and medically guided approach that prioritizes safety, integration, and long-term recovery over quick fixes.